Subscribe to our newsletter
RAPID DETECTION OF ENTAMOEBA HISTOLYTICA FROM HUMAN FECAL SPECIMENS
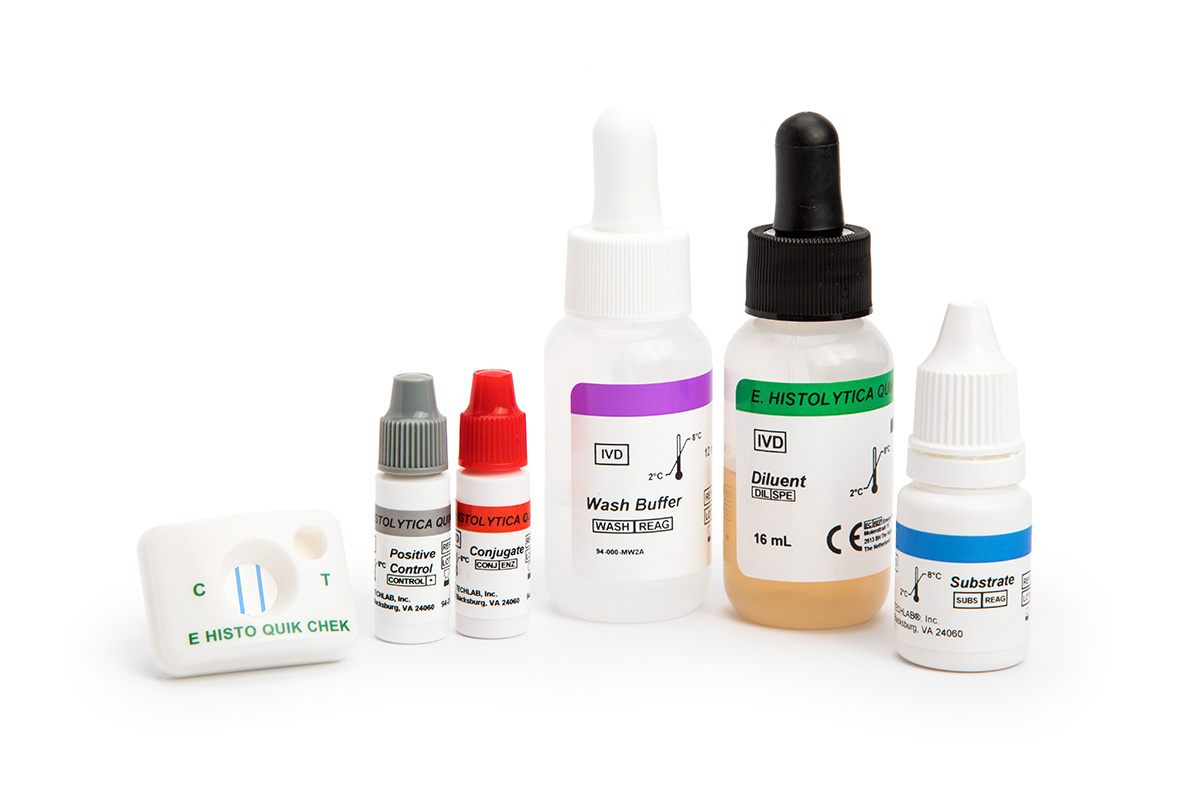
The E. HISTOLYTICA QUIK CHEK™ test is a rapid membrane enzyme immunoassay for the qualitative detection of Entamoeba histolytica in a single use cassette. It is an aid in the diagnosis of E. histolytica gastrointestinal infection.
This kit is intended for use with human fecal specimens from patients with diarrhea or dysentery and is not suitable for use with preserved human fecal specimens.
TECHLAB® E. HISTOLYTICA QUIK CHEK™
- Results in approximately 30 minutes
- No instrumentation required
- Single test cassette format
- Does not cross react with non-pathogenic E. dispar,
- Does not cross react with Entamoeba coli, E moshkovski or E. bangladeshi
- Performance Characteristics (compared to a composite reference method including molecular detection)
- Sensitivity 40.0%
- Specificity 100%
- Limit of Detection (LOD) pathogenic zymodemes (PZs)/ml
- 320 PZs/ml fresh fecal sample
- 275 PZs/ml Cary Blair media
- 245 PZs/ml C&S media
E.histolytica is an intestinal parasite that infects approximately a half billion persons worldwide annually, about 10% become symptomatic and develop colitis and liver abscesses, resulting in a mortality rate estimated between 40,000 and 120,000 persons a year.
Patients infected with pathogenic E. histolytica may show a wide range of conditions. Some may be asymptomatic; others may show mild diarrhea that develops into bloody diarrhea and eventually fulminant colitis.
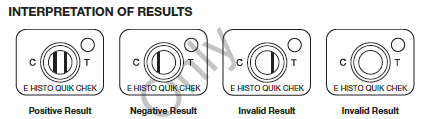
The Techlab Quik Chek System principle
Ordering Details
Product Code: TLI-T30409 E. HISTOLYTICA QUIK CHEK® (25 tests)